
Nuclear Fusion

Nuclear Fusion



Nuclear FissionIf a massive nucleus like uranium-235 breaks apart (fissions), then there will be a net yield of energy because the sum of the masses of the fragments will be less than the mass of the uranium nucleus. If the mass of the fragments is equal to or greater than that of iron at the peak of the binding energy curve, then the nuclear particles will be more tightly bound than they were in the uranium nucleus, and that decrease in mass comes off in the form of energy according to the Einstein equation. For elements lighter than iron, fusion will yield energy.The fission of U-235 in reactors is triggered by the absorption of a low energy neutron, often termed a "slow neutron" or a "thermal neutron". Other fissionable isotopes which can be induced to fission by slow neutrons are plutonium-239, uranium-233, and thorium-232. 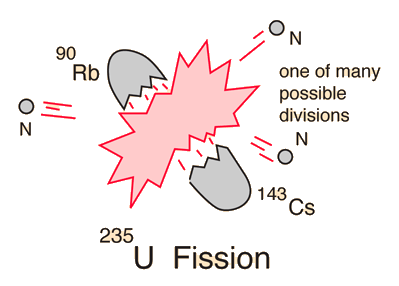
| Index Fission concepts | |||||||||||||
| Go Back |
Uranium-235 Fission
| Index Fission concepts | ||||||
| Go Back |
Uranium FuelNatural uranium is composed of 0.72% U-235 (the fissionable isotope), 99.27% U-238, and a trace quantity 0.0055% U-234 . The 0.72% U-235 is not sufficient to produce a self-sustaining critical chain reaction in U.S. style light-water reactors, although it is used in Canadian CANDU reactors. For light-water reactors, the fuel must be enriched to 2.5-3.5% U-235.
| Index | ||
| Go Back |
Fissionable IsotopesWhile uranium-235 is the naturally occuring fissionable isotope, there are other isotopes which can be induced to fission by neutron bombardment. Plutonium-239 is also fissionable by bombardment with slow neutrons, and both it and uranium-235 have been used to make nuclear fission bombs. Plutonium-239 can be produced by "breeding" it from uranium-238. Uranium-238, which makes up 99.3% of natural uranium, is not fissionable by slow neutrons. U-238 has a small probability for spontaneous fission and also a small probability of fission when bombarded with fast neutrons, but it is not useful as a nuclear fuel source. Some of the nuclear reactors at Hanford, Washington and the Savannah-River Plant (SC) are designed for the production of bomb-grade plutonium-239. Thorium-232 is fissionable, so could conceivably be used as a nuclear fuel. The only other isotope which is known to undergo fission upon slow-neutron bombardment is uranium-233.
"Splitting the atom" redirects here. For the EP, see Splitting the Atom.
 An induced fission reaction. A slow-moving neutron is absorbed by a uranium-235 nucleus turning it briefly into a uranium-236 nucleus; this in turn splits into fast-moving lighter elements (fission products) and releases three free neutrons. In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which thenucleus of an atom splits into smaller parts (lighter nuclei), often producing free neutrons andphotons (in the form of gamma rays), and releasing a tremendous amount of energy. The two nuclei produced are most often of comparable size, typically with a mass ratio around 3:2 for commonfissile isotopes.[1][2] Most fissions are binary fissions, but occasionally (2 to 4 times per 1000 events), three positively-charged fragments are produced in a ternary fission. The smallest of these ranges in size from a proton to an argon nucleus. Fission is usually an energetic nuclear reaction induced by a neutron, although it is occasionally seen as a form of spontaneous radioactive decay, especially in very high-mass-number isotopes. The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products every time. Fission of heavy elements is an exothermic reaction which can release large amounts of energyboth as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be greater than that of the starting element. Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. Nuclear fission produces energy for nuclear power and to drive the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon. The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over thedestructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power. |